| STANDARD ELECTRODE POTENTIALS
| Half-Reaction
|
Eo (V) |
| Li+ + e- → Li |
-3.04 |
| K+ + e- → K |
-2.92 |
| Ba2+ + 2e- → Ba |
-2.90 |
| Ca2+ + 2e- → Ca |
-2.87 |
| Na+ + e- → Na |
-2.71 |
| Mg2+ + 2e- → Mg |
-2.37 |
| Al3+ + 3e- → Al |
-1.66 |
| Mn2+ + 2e- → Mn |
-1.18 |
| 2H2O + 2e- → H2(g) + 2 OH- |
-0.83 |
| Zn2+ + 2e- → Zn |
-0.76 |
| Cr2+ + 2e- → Cr |
-0.74 |
| Fe2+ + 2e- → Fe |
-0.44 |
| Cr3+ + 3e- → Cr |
-0.41 |
| Cd2+ + 2e- → Cd |
-0.40 |
| Co2+ + 2e- → Co |
-0.28 |
| Ni2+ + 2e- → Ni |
-0.25 |
| Sn2+ + 2e- → Sn |
-0.14 |
| Pb2+ + 2e- → Pb |
-0.13 |
| Fe3+ + 3e- → Fe |
-0.04 |
| 2H+ + 2e-→ H2(g) |
0.00 |
| S + 2H+ + 2e-→ H2S(g) |
0.14 |
| Sn4+ + 2e- → Sn2+ |
0.15 |
| Cu2+ + e- → Cu+ |
0.16 |
| SO42+ + 4H+ + 2e-→ SO2(g) + 2H2O |
0.17 |
| Cu2+ + 2e- → Cu |
0.34 |
| 2H2O + O2 + 4e- → 4OH- |
0.40 |
| Cu+ + e- → Cu |
0.52 |
| I2 + 2e- → 2I- |
0.54 |
| O2 (g) + 2H+ + 2e- → H2O2 |
0.68 |
| Fe3+ + e- → Fe2+ |
0.77 |
| NO3- + 2H+ + e-→ NO2 (g) + H2O |
0.78 |
| Hg2+ + 2e- → Hg (l) |
0.78 |
| Ag+ + e- → Ag |
0.80 |
| NO3- + 4H+ +3 e-→ NO (g) + 2H2O |
0.96 |
| Br2 + 2e- → 2Br- |
1.06 |
| O2 (g) + 4H+ + 4e- → 2H2O |
1.23 |
| MnO2 + 4H+ + 2e- → Mn2+ + 2H2O |
1.28 |
| Cr2O72- + 14H+ + 6e- → 2Cr3+ + 7H2O |
1.33 |
| Cl2 + 2e- → 2Cl- |
1.36 |
| Au3+ + 3e- → Au |
1.50 |
| MnO4- + 8H+ + 5e-→ Mn2+ + 4H2O |
1.52 |
| Co3+ + e- → Co2+ |
1.82 |
| F2 + 2e- → 2F- |
2.87 |
| |
Determining Standard State Cell Potentials
To calculate the standard cell potential for a reaction
- Write the oxidation and reduction half-reactions for the cell.
- Look up the reduction potential, E0reduction, for the reduction half-reaction in a table of reduction potentials.
- Look up the reduction potential for the reverse of the oxidation half-reaction and reverse the sign to obtain the oxidation potential. For the oxidation reaction,
E0oxidation = -E0reduction
- Add the potentials of the half-cells to get the overall standard cell potential:
E0cell = E0reduction + E0oxidation
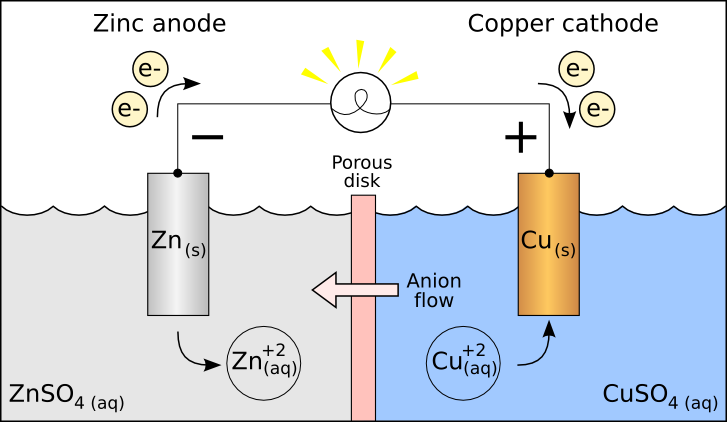
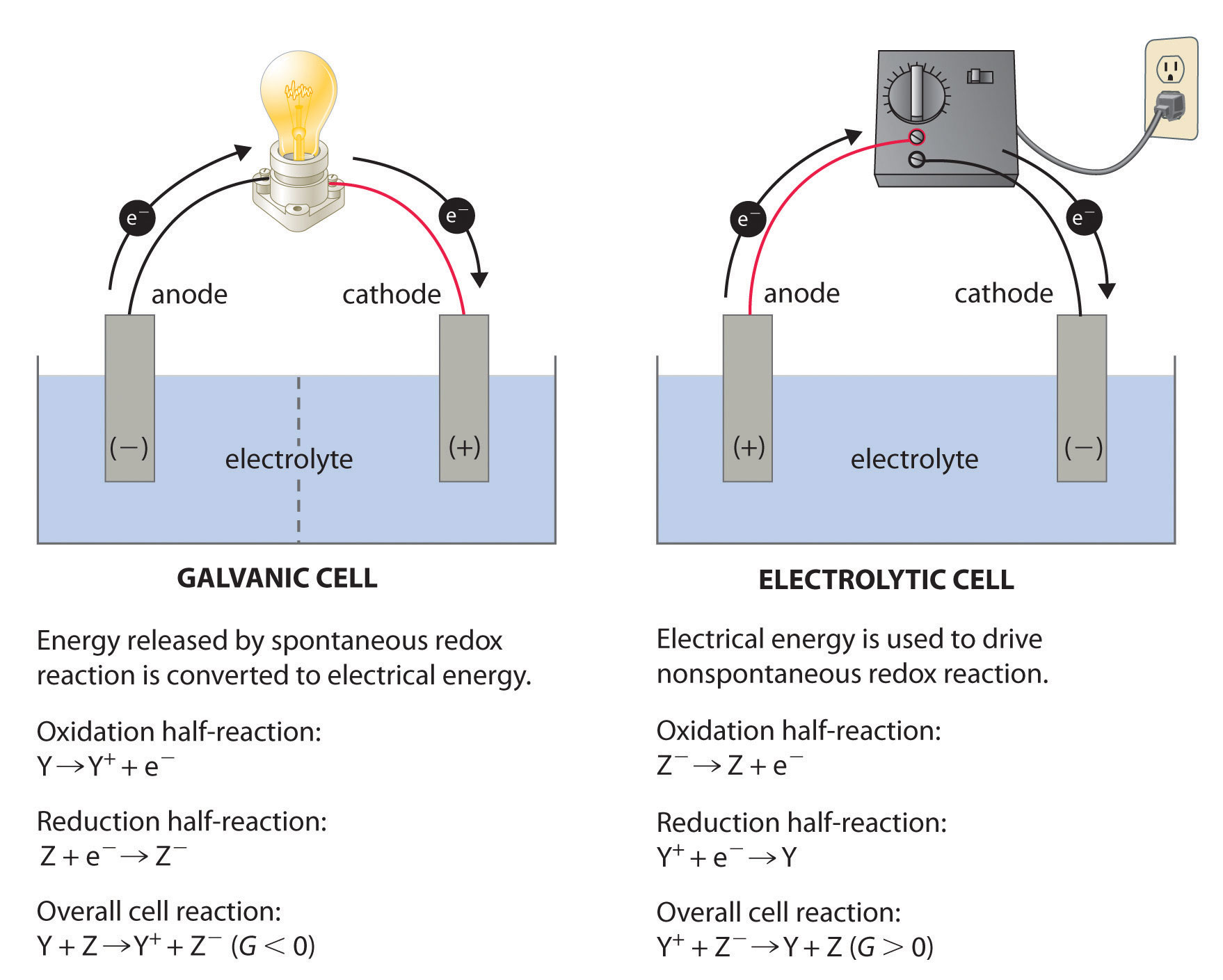
Flow of Electrons
Electrons always flow from the anode to the cathode, or from the oxidation half cell to the reduction half cell. In terms of the E0 of the half reactions, the electrons will flow from the more negative half reaction to the more positive half reaction.
Losing Electrons is Oxidation, Gaining Electrons is Reduction
OIL RIG: Oxidation Is Loss, Reduction Is Gain
Reduction at the Cathode, Anode for Oxidation
Oxidizing Agent = electron acceptor; Reducing agent = electron donor
For oxidation half reaction, the e- is on the right side. It's on the left for reductions.
Strongest reducing agent (top of chart), most likely to be oxidized, will lose e-
Strongest oxidizing agent (bottom of list), most likely to be reduced, will gain e-
Rules for Assigning Oxidation States
- The oxidation state of an atom in an uncombined element is 0.
- The oxidation state of a monatomic ion is the same as its charge.
- Oxygen is assigned an oxidation state of -2 in most of its covalent compounds. Important exception: peroxides (compounds containing the O22- group), in which each oxygen is assigned an oxidation state of -1.
- In its covalent compounds with nonmetals, hydrogen is assigned an oxidation state of -1.
- In binary compounds, the element with the greater electronegativity is assigned a negative oxidation state equal to its charge as an anion in its ionic compounds.
- For an electrically neutral compound, the sum of the oxidation states must be zero.
- For an ionic species, the sum of the oxidation states must equal the overall charge.
|
Galvanic cell example
|
|

